




The treatment landscape for metastatic castration-resistant prostate cancer (mCRPC) has continued to evolve over the past decade, with the approval of many drugs with diverse mechanisms of action that can provide a survival benefit. The advent of radiopharmaceuticals in particular has had a significant impact on the treatment of patients with mCRPC and bone metastases. While several such agents have been approved, only two, radium-223 dichloride (Ra-223) and lutetium-177–labeled PSMA-617 (LuPSMA), have been shown to significantly improve survival.
Ra-223, a first-in-class alpha-particle emitter, targets bone metastases in mCRPC. Preclinical studies have demonstrated its preferential incorporation into areas of increased bone turnover, allowing for delivery of intense, highly localized radiation dose to osteoblastic metastases with fewer effects on healthy bone. In clinical trials, Ra-223 therapy resulted in prolonged survival as well as a reduction in bone pain, fewer skeletal-related events, and improved quality of life.1 Approved by the U.S. Food and Drug Administration (FDA) in 2013 for use in patients with mCRPC with bone metastases and no known visceral metastases, it is the only alpha-emitter that has been shown to significantly improve survival.2 Ra-223 potentially has broad use since bone is a common site for CRPC metastasis, and approximately 90% of patients develop bone metastases over the course of their disease.3 This life-prolonging therapy has now been used in clinical practice for the past 10 years following FDA approval, with subsequent regulatory approval by more than 50 other countries worldwide. Ra-223 has been adopted as a standard of care for mCRPC with bone metastases and is now included in all prostate cancer guidelines.4-7 Ra-223 may be underutilized, however, due to various reasons such as a lack of an accompanying prostate-specific antigen (PSA) response and a preference by some clinicians to rechallenge with an androgen receptor (AR) inhibitor upon disease recurrence, despite a lack of evidence on the effectiveness of this approach.5,6,8 Ongoing clinical trials (discussed below) are investigating Ra-223–based combination regimens and in its use in other patient populations to potentially broaden its application.
Another radiopharmaceutical, LuPSMA, has been approved for patients with prostate-specific membrane antigen (PSMA)-positive mCRPC who have received a novel AR pathway inhibitor (eg, abiraterone, enzalutamide) and taxane-based chemotherapy.9 In contrast to Ra-223, LuPSMA is a beta-particle emitter and delivers radiation to PSMA-positive tumors and the surrounding microenvironment.
In the first published phase II trial, Ra-223 treatment of patients with mCRPC and symptomatic bone metastases resulted in a longer time to first skeletal-related events compared with placebo.10 The pivotal phase III randomized trial, ALSYMPCA, compared Ra-223 to placebo in patients with mCRPC and symptomatic bone metastases but no visceral metastases. Of the 921 patients enrolled, 59% received all six planned Ra-223 injections. Ra-223 significantly improved median overall survival (OS) compared with placebo (14.9 months vs 11.3 months; hazard ratio = 0.70, 95% confidence interval = 0.58–0.83; P < .001), reflecting a 30% reduction in risk of death. The survival benefit of Ra-223 was consistent across all subgroups.11,12 Moreover, Ra-223 was equally effective in docetaxel-treated and docetaxel-naive patients.13 Other secondary efficacy endpoints, such as time to first skeletal event and reduction in PSA, also significantly improved with Ra-223.
The overall incidence of all-grade adverse events was lower with Ra-223 than placebo (93% vs 96%) as were grade 3/4 adverse events (56% vs 62%) and serious adverse events (47% vs 60%). The most common all-cause adverse events (> 20%) with Ra-223 treatment were bone pain (50%), nausea (36%), anemia (31%), fatigue (26%), diarrhea (25%). Patient quality of life, as measured by FACT-P total score and EQ-5D, was significantly better with Ra-223 than placebo.14,15 Moreover, Ra-223 treatment was associated with a lower hospitalization rate and fewer hospitalization days,14 which could contribute to lower health-care resource utilization as demonstrated in a cost-effectiveness model.16
The results of the ALSYMPCA trial led to FDA approval of Ra-223 for treatment of patients with mCRPC, symptomatic bone metastases detected on conventional imaging and no known visceral metastatic disease. A 3-year safety follow-up study revealed no new safety issues.17 Data from subsequent studies support the improvement in disease-related quality of life with Ra-223 observed in ALSYMPCA, as well as pain relief from bone metastases.18
According to Oliver Sartor, MD, Director of Radiopharmaceutical Trials, Mayo Clinic, Rochester, “Radium-223 was the first and only alpha-emitter approved by regulatory agencies. It was the first bone-targeted treatment that was able to prolong survival. That's a very important landmark, and I think it surprised a lot of people who didn't think that a bone-targeted agent would be able to prolong survival.”
Radium-223 was the first and only alpha-emitter approved by regulatory agencies. It was the first bone-targeted treatment that was able to prolong survival. That's a very important landmark, and I think it surprised a lot of people who didn't think that a bone-targeted agent would be able to prolong survival. Oliver Sartor, MD
Multiple real-world studies have confirmed and extended the benefits of Ra-223 seen in the ALSYMPCA trial and have identified approaches to further optimize efficacy. A review of more than 40 real-world studies of Ra-223 for mCRPC found that results generally supported the efficacy observed in ALSYMPCA, with OS ranging from 8.2 months to 29 months.6 These studies indicated that survival depends on multiple factors including patient characteristics, completion of all six Ra-223 treatment cycles, other therapies used, and when Ra-223 is used during treatment. They also suggest that use of Ra-223 in asymptomatic or minimally symptomatic patients may better allow completion of planned therapy and maximize benefits compared with its use in symptomatic patients.19
In one study, a higher proportion of asymptomatic patients completed Ra-223 compared with symptomatic individuals; asymptomatic patients also had superior OS, time to disease progression, and time to first symptomatic skeletal events.20 A retrospective study supported the potential benefits of earlier use of Ra-223, since docetaxel-naive patients had a significantly lower all-cause mortality rate and higher treatment completion rate than those who had previously received docetaxel therapy.21 Use of Ra-223 prior to chemotherapy may therefore favor completion of all planned cycles of Ra-223, which can result in prolonged survival. Another study found that men with symptomatic bone metastases who experienced disease progression following an initial course of Ra-223 could be safely re-treated with this agent, resulting in a 2-year median radiographic progression-free survival of 9.9 months and median OS of 24.4 months.22 Moreover, Ra-223 appears to have comparable efficacy in older and younger patients (≥ 72 years old vs < 72 years).23
The efficacy of Ra-223 in these retrospective studies is supported by initial results from the ongoing global, prospective, observational real-world study of Ra-223 for patients with mCRPC and bone metastases (REASSURE). At a prespecified interim analysis (median follow-up of nearly 12 months), the median OS was 17.8 months.24,25 Ra-223 efficacy did not appear to be impacted by prior, concomitant, or subsequent use of other therapies. The most common (> 5%) any-grade drug-related treatment-related adverse events were diarrhea (10%), fatigue (9%), anemia (8%), and nausea (7%), with 10% grade ≥ 3 drug-related treatment-related adverse events. The incidence of second primary malignancies during or after Ra-223 therapy was low (1%), similar to the ALSYMPCA trial. Final results of the REASSURE study are expected in 2024.
The safety and efficacy of concomitant use of Ra-223 with docetaxel or other systemic therapy (except androgen-deprivation therapy) has not been fully assessed and is not recommended.7 Ra-223 should not be used with abiraterone/prednisone, since this regimen increased risk of fractures and did not improve symptomatic skeletal event-free survival compared with abiraterone/prednisone/placebo in the ERA 223 trial.26 While the risk of bone fractures with Ra-223 monotherapy does not appear to significantly differ from that of other standard mCRPC therapies, use of denosumab or zoledronic acid is recommended to maintain bone health.27
The tolerable safety profile and limited myelosuppression of Ra-223 facilitates its use in combination with other agents. Several ongoing clinical trials are evaluating Ra-223 in combination with chemotherapy, immunotherapy, and targeted agents (Table 1). The combination of Ra-223 and enzalutamide, for example, has demonstrated safety and promising efficacy in men with mCRPC, supporting further evaluation of this regimen in the ongoing PEACE III trial.28
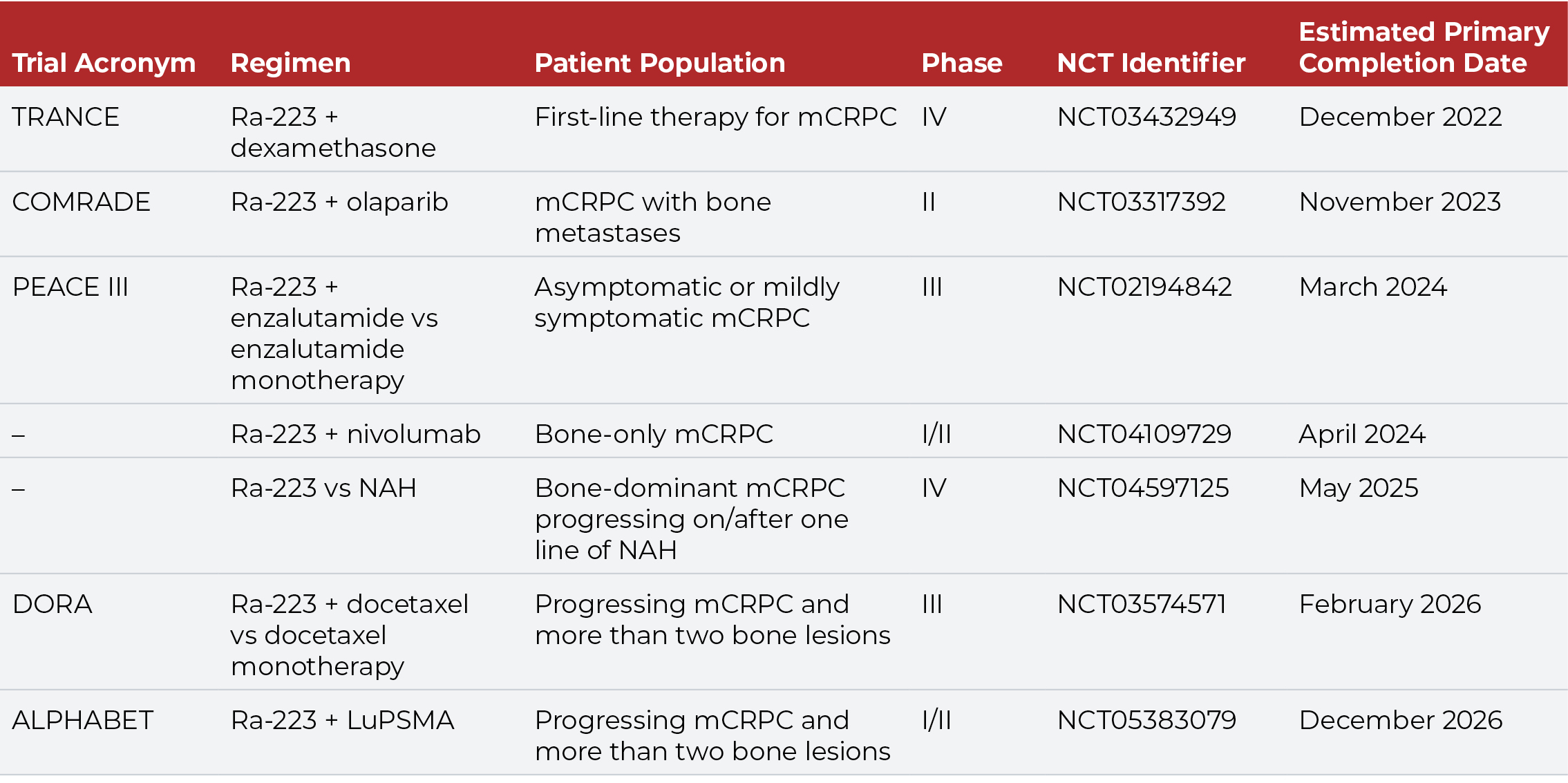
While Ra-223 is indicated for patients with bone-only or bone-predominant disease, some studies suggest that earlier use of Ra-223 in the mCRPC treatment pathway also may improve survival.29,30 Overall survival is greater when Ra-223 is used in patients with one to two prior lines of therapy compared with three or more lines of therapy. Moreover, outcomes are better when Ra-223 is administered prior to rather than folllowing chemotherapy. Use of Ra-223 prior to chemotherapy increases the probability of completing all six courses of this radiopharmaceutical, and completion of all six cycles of Ra-223 is associated with improved survival.21,31-34 Prior myelosuppression due to chemotherapy is associated with poor OS, and patients with a better baseline hematologic profile are more likely to complete Ra-223 therapy.34
Other data also support consideration of Ra-223 therapy earlier in the mCRPC disease course since survival is superior when used in patients with minimally symptomatic disease compared with more symptomatic disease. Because visceral metastases can occur even in early-stage mCRPC, Ra-223 might be initiated sooner rather than later, before symptoms become clinically relevant or severe. Once a patient develops visceral metastases (the probability of which increases over time), Ra-223 is no longer an approved treatment option. Additionally, Ra-223 might provide clinical benefits for patients who do not have severe bone pain. In one study of men with extensively pretreated symptomatic or asymptomatic mCRPC who received Ra-223, the majority experienced clinically meaningful reduction in pain, decreased opioid use, and improved quality of life. These results suggest that clinicians should consider the potential benefits of a broader spectrum of symptom endpoints such as fatigue, discomfort, and quality of life when considering use of Ra-223, and not only severe bone pain.35 “Use of Ra-223 in asymptomatic patients seems justifiable since it’s very well tolerated by most patients,” noted Dr. Sartor. “Also, in contrast to LuPSMA, with Ra-223 there is no requirement for prior chemotherapy or androgen receptor pathway inhibitors, so the opportunity to use radium early is expanded by the current label.”
Studies indicate the importance of addressing patient preference when considering treatment options for metastatic prostate cancer. In a recent survey of U.S. patients with mCRPC, improving OS was ranked as the highest priority, followed by controlling bone pain, nausea, and delaying fracture or bone metastasis.36 Notably, respondents were willing to trade some survival benefit to reduce the risk of severe nausea and fatigue, delaying fracture or bone metastasis, and requiring radiation to control bone pain. Another study found that expected efficacy and pain control were the most important factors in selecting therapy.37 Similarly, Japanese men with mCRPC scheduled to receive Ra-223 were most concerned with relief from disease-related pain, quality of life, and anxiety about disease progression.38 These patients had high expectations for Ra-223 treatment to halt disease progression and alleviate pain. Good communication between patients and providers can help address patient expectations and concerns regarding the efficacy and safety of bone-targeted radiopharmaceuticals like Ra-223 for treatment of mCRPC.
In contrast to Ra-223, LuPSMA is a beta-emitter that selectively binds to PSMA receptors on prostate cancer cells. In clinical trials, LuPSMA was shown to improve response rate and survival compared with standard of care in men with PSMA-expressing mCRPC previously treated with an AR pathway inhibitor and one to two taxane regimens.39-41 These results led to approval of LuPSMA for treatment of this patient population.9 “The PSMA targeting moiety for LuPSMA allows it to target both bone and soft tissue, whereas for Ra-223, the radium only allows it to target bone,” noted Benjamin Maughan, MD, PharmD, of Huntsman Cancer Institute in Salt Lake City. While its use is not restricted to patients with bone-only metastases like Ra-223, it does have limitations. Because LuPSMA is approved only for patients with PSMA-positive tumors, a positron-emission tomography (PET) scan using a gallium-68–labeled PSMA-11 imaging agent is required to determine PSMA expression and patient eligibility. (This is distinct from other imaging techniques commonly used for bone and soft tissue scans such as F-18 piflufolastat PSMA PET/CT or PET/MRI.) In contrast with Ra-223, use of LuPSMA requires that patients must have previously received AR pathway inhibition and taxane-based chemotherapy. The risk of radiation exposure with LuPSMA requires shielding during administration, and the potential exists for radiotoxicity to salivary glands, kidneys, and other PSMA-positive organs. Importantly, up to 30% of patients are nonresponders or develop resistance to LuPSMA and experience disease progression.42
Dr. Maughan noted that both of these radiopharmaceuticals can have bone marrow suppressive effects, which can be relatively limited or very profound. “Radiation exposure to bone marrow may depend on whether a patient has a relatively higher or lower density of bone metastases,” he observed. “Patients with a higher number of bone metastases have much more significant effects on their bone marrow.”
Radiation exposure to bone marrow may depend on whether a patient has a relatively higher or lower density of bone metastases. Patients with a higher number of bone metastases have much more significant effects on their bone marrow. Benjamin Maughan, MD, PhD
Ra-223 and LuPSMA act through distinct mechanisms of action, and both can prolong survival in mCRPC; however, the optimal sequencing of these agents is unclear. Some studies have demonstrated the feasibility of LuPSMA therapy following progression on Ra-223, without adversely affecting the survival benefit.43-46 For example, a retrospective study (RALU) evaluated sequential therapy with Ra-223 and LuPSMA in patients with mCRPC. Administration of LuPSMA either < 6 months or ≥ 6 months following the last dose of Ra-223 resulted in a median OS (from start of LuPSMA) of 12.0 months and 13.2 months, respectively. During LuPSMA therapy, PSA response ≥ 50% occurred in 53% and 39% of patients. The rate of treatment-emergent adverse events was comparable on both arms, as were treatment delays and treatment-related deaths.46 These results suggest that use of LuPSMA within 6 months of completing Ra-223 is feasible and well tolerated, with survival rates similar with earlier or later use of LuPSMA. The ongoing ALPHABET trial is also investigating whether combining these two agents can further enhance efficacy and improve eradication of micrometastases.47
At present, there are no biomarkers that can reliably predict response to Ra-223. Factors associated with improved OS include PSA doubling time, change in neutrophil-to-lymphocyte ratio during treatment, early alkaline phosphatase dynamics, baseline metabolic tumor burden and systemic inflammation, and circulating tumor cells.48-55 Younger age, better performance status, lower disease burden, and less prior chemotherapy are also associated with improved survival, as is low baseline bone scan index.54,56 Ongoing studies are evaluating these and other novel predictive biomarkers that could improve selection of patients for Ra-223 therapy.
While the treatment landscape for mCRPC has evolved significantly since approval of Ra-223 a decade ago, clinical use of this radiopharmaceutical has continued to expand. Multiple real-world studies of Ra-223 have confirmed and supported findings of the pivotal ALSYMPCA trial, making these results more generalizable to broader patient populations. Ongoing trials are continuing to build on this experience and are exploring novel combinations and sequencing to optimize its use and potentially expand its therapeutic benefit for treatment of mCRPC.
Dr. Sartor reported stock and other ownership interests with AbbVie, Cardinal Health, Clarity Pharmaceuticals, Clovis, GlaxoSmithKline, Lilly, Noria Therapeutics, Inc, PSMA Therapeutics, and United Health Group; has served in a consulting or advisory role for Advanced Accelerator Applications, ARTbio, Astellas Pharma, AstraZeneca, Bavarian Nordic, Bayer, Blue Earth Diagnostics, Bristol Myers Squibb, Clarity Pharmaceuticals, Clovis, Constellation Pharmaceuticals, Dendreon , EMD Serono, Fusion, Hengrui, Isotopen Technologien Meunchen, Janssen, Merck, Morphimmune, Myovant, Myriad Genetics, Noria Therapeutics, Northstar, Novartis, Noxopharm, Pfizer, Point Biopharma, Progenics, Sanofi, Telix, Tempus, TeneoBio, Tessa Therapeutics, and Theragnostics; has received research funding from Advanced Accelerator Applications, Amgen, AstraZeneca, Bayer, Constellation Pharmaceuticals, Dendreon, Endocyte, InVitae, Janssen, Lantheus, Merck, Progenics, Sanofi, and SOTIO; reported a patent for Saposin C and receptors as targets for treatment of benign and malignant disorders (U.S. patent awarded January 23, 2007; patent no. 7,166,691); has provided expert testimony for Sanofi; and reported travel, accommodations, or expenses from AstraZeneca, Bayer, Johnson & Johnson, Progenics, and Sanofi.
Dr. Maughan has served in a consulting or advisory role for Astellas Medivation, AVEO Oncology, Bavarian Nordic, Bayer, Bristol Myers Squibb, Clovis, Exelixis, Janssen Oncology, Merck, Peloton Therapeutics, Pfizer, and Tempus; has received research funding from Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, and Exelixis; and reported travel, accommodations, or expenses from Exelixis.
Sponsored content is not written by and does not necessarily reflect the views of ASCO or The ASCO Post editorial staff. It is authored by Harborside Studio writers or independent medical writers approved by Harborside Studio. Harborside Studio's sponsored content is held to editorial standards expected in The ASCO Post with the intent to provide valuable information to The ASCO Post readers. The mention of any company, product, service, or therapy does not constitute an endorsement of any kind by ASCO. ASCO assumes no responsibility for any injury or damage arising out of or related to use of the sponsored content or any errors or omissions. This sponsored content has been produced with funding support from Bayer.